Ireland Soil and Water samples tested in a Lab
The Mass Murdering "factcheckers" are there to prevent exposure to a serious attack by the Globalist "New World Order" Payco-killers.
Result of Soil and Water samples taken in Ireland. These samples were scientifically analyzed at the address below:
Address: Boyne Business Park, Unit 35A,, Drogheda, Co. Louth Phone: (041) 984 5440
Anna Harvey, who handed in the samples, writes - (full article - here)
These are the effects of the toxic chemicals found to be falling on our people being absorbed into our skin and into our bloodstream by chemicals they are spraying in the sky. I was curious to see what they were spraying and I had some water and soil samples taken and sent to the lab for analysis to see what they were. I had come across an article in America but I do not believe in speculation I just deal with the facts so I needed proof and the results you will see below for your self and the side effects are devastating.
This is Fitz Science Lab in Drogheda where the Samples were conducted, everything was done professionally.
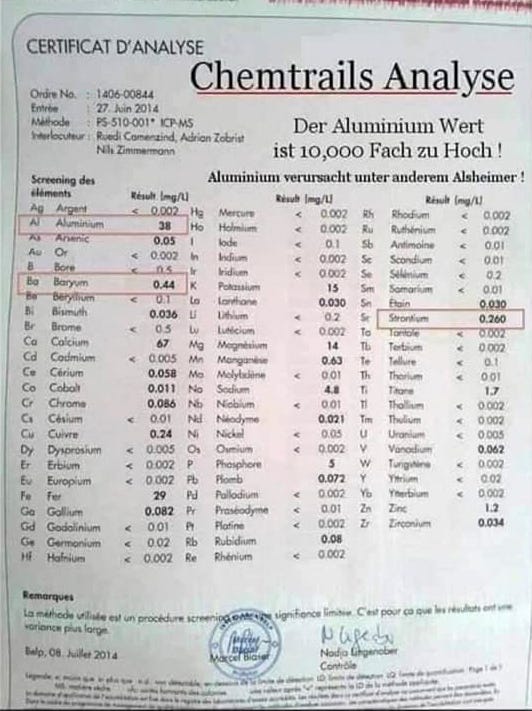
The soil samples revealed A reading of
Aluminium ……1306584.00 … ug/kg ….off the scales
Barium ……….. 1188.31…. ug/kg.
Lithium ……… < 10 ug/kg
Strontium 90 ……… 4726.93 ug/kg
That amount was present in an inch of soil taken from the bog as it preserves everything that is falling on our people. That amount is staggering for the amount in just an inch and if you do your research it is strontium 90 that they spray from the planes
The Water Sample revealed
Taken from the Donegal Co Council Reservoir
Chemical Sop 177…Pvl ……… Results ug/L
The Water Sample revealed
Taken from the Donegal Co Council Reservoir
Chemical Sop 177-------- Pvl ……… Results ug/L
Aluminium -----------------200-----------------147.8
Antimony -------------------5 -------------------0.243
Arsenic --------------------10 --------------------0.96
Barium -------------------------------------------2.978
Beryllium-------------------------------------------0.20
Boron -----------------------1--------------------0.011
Cadmium -------------------5---------------------0.09
Cosium------------------------------------------- < 0.37
Cobalt-----------------------------------------------<0.2
Copper----------------------2 -------------------0.0004
Gallium ------------------------------------------- < 0.32
Iron-------------------------200--------------------- 50.3
Lead--------------------------10--------------------- 0.38
Lithium----------------------------------------------<3.32
Manganese ---------------50----------------------25.14
Nickel -----------------------20------------------- <0.47
Rubidium ------------------------------------------1.313
Selenium -------------------10 --------------------0.909
Silver -----------------------------------------------<0.33
Strontium 90 --------------------------------------8.724
Thallium -------------------------------------------< 0.2
Tin --------------------------------------------------< 2.8
Uranium __________________________ < 0.93
Vanadium _---------------------------------------- 4.0
Zinc ------------------------------------------- < 4.6
SOIL SAMPLES SHOW; results :
Aluminum: 1306584.00
Barium: 1188.31
Lithium: < 10
Strontium: 4726.93
WATER SAMPLES SHOW; Results;
PVL MEANS Parametric Value Limit as per EU Drinking water regulations
Aluminium:
Pvl =200.. results … 147.8 ug/L
By Rebel Siren
Alzheimer’s Disease Now Fastest-Growing Threat Globally Health, Report Finds
Source: by Melvyn R. Werbach, M.D.
STUDIES HAVE DISCOVERED A DIRECT ASSOCIATION BETWEEN THE
HIGH LEVELS ALUMINUM IN MUNICIPAL DRINKING WATER AND THE ONSET OF ALZHEIMERS
DEMENTIA” which has multiplied 80% in the past few years
Antimony: Pvl =5..Results =0.243 ug/L
Health Effects of Antimony
Especially people who work with antimony can suffer the effects of exposure by breathing in antimony dust. Human exposure to antimony can take place by breathing air, drinking water, and eating foods that contain it, but also by skin contact with soil, water, and other substances that contain it. Breathing in antimony that is bonded to hydrogen in the gaseous phase, is what mainly causes the health effects.
Exposure to relatively high concentrations of antimony (9 mg/m3 of air) for a longer time can irritate the eyes, skin, and lungs.
As the exposure continues more serious health effects may occur, such as lung diseases, heart problems, diarrhea, severe vomiting, and stomach ulcers.
It is not known whether antimony can cause cancer or reproductive failure. Antimony is used as a medicine for parasital infections, but people who have had too much of the medicine or were sensitive to it have experienced health effects in the past. These health effects have made us more aware of the dangers Especially people who work with antimony can suffer the effects of exposure by breathing in antimony dust. Human exposure to antimony can take place by breathing air drinking water and eating foods that contain it, but also by skin contact with soil water and other substances that contain it of exposure to antimony.
https://en.wikipedia.org/wiki/Antimony
Arsenic:
Arsenic is Arsenic, IT CAN KILL YOU.
Arsenic can cause various health effects, such as irritation of the stomach and intestines, decreased production of red and white blood cells, skin changes and lung irritation. It is suggested that the uptake of significant amounts of inorganic arsenic can intensify the chances of cancer development, especially the chances of development of skin cancer, lung cancer, liver cancer, and lymphatic cancer.
Very high exposure to inorganic arsenic can cause infertility and miscarriages in women, and it can cause skin disturbances, declined resistance to infections, heart disruptions, and brain damage in both men and women.
Finally, inorganic arsenic can damage DNA.
A lethal dose of arsenic oxide is generally regarded as 100 mg.
Organic arsenic…?... can cause neither cancer nor DNA damage. However, exposure to high doses may cause certain effects on human health, such as nerve injury and stomachaches. Arsenic is Arsenic prolonged exposure kills and causes serious damage
Chemical properties of arsenic - Health effects of arsenic - Environmental effects of arsenic
http://www.lenntech.com/periodic/elements/as.htm...
Barium:
Barium causes stomach cramps diarrhea, nausea, vomiting, constipation, weakness, pale skin, sweating, ringing in the ears, hives, itching, red skin, swelling or tightening of the throat, difficulty breathing or swallowing, hoarseness
agitation, confusion, fast heartbeat, bluish skin
Chemical properties of barium - Health effects of barium - Environmental effects of barium
https://www.lenntech.com/periodic/elements/ba.htm
Beryllium:
Health effects of beryllium
Beryllium is not an element that is crucial for humans; in fact it is one of the most toxic chemicals we know. It is a metal that can be very harmful when humans breathe it in because it can damage the lungs and cause pneumonia.
The most commonly known effect of beryllium is called berylliosis, a dangerous and persistent lung disorder that can also damage other organs, such as the heart. In about 20% of all cases, people die of this disease. Breathing in beryllium in the workplace is what causes berylliosis. People who have weakened immune systems are most susceptible to this disease.
Beryllium can also cause allergic reactions in people who are hypersensitive to this chemical. These reactions can be very heavy and they can even cause a person to be seriously ill, a condition known as Chronic Beryllium Disease (CBD). The symptoms are weakness, tiredness, and breathing problems. Some people who suffer from CBD will develop anorexia and blueness of hands and feet. Sometimes people can even be in such a serious condition that CBD can cause their death.
Next to causing berylliosis and CBD, beryllium can also increase the chances of cancer development and DNA damage.
Chemical properties of beryllium - Health effects of beryllium - Environmental effects of beryllium
https://www.lenntech.com/periodic/elements/be.htm
Boron:
Health effects of boron
Humans can be exposed to boron through fruit and vegetables, water, air, and consumer products. we have a regular daily intake of about 2 mg and about 18 mg in our body in total.
When humans consume large amounts of boron-containing food, the boron concentrations in their bodies may rise to levels that can cause health problems. Prolonged exposure to Boron can infect the stomach, liver, kidneys, and brains and can eventually lead to death. When exposure to small amounts of boron takes place irritation of the nose, throat, or eyes may occur. It takes 5 g of boric acid to make a person ill and 20 grams or more to put their life in danger.
Eating fish or meat will not increase the boron concentrations in our bodies, as boron does not accumulate within the tissues of animals.
Chemical properties of boron - Health effects of boron - Environmental effects of boron
http://www.lenntech.com/periodic/elements/b.htm...
Cadmium:
Other high exposures can occur with people who live near hazardous waste sites or factories that release cadmium into the air and people who work in the metal refinery industry. When people breathe in cadmium it can severely damage the lungs. This may even cause death.
Cadmium is first transported to the liver through the blood. There, it is bonded to proteins to form complexes that are transported to the kidneys. Cadmium accumulates in the kidneys, where it damages filtering mechanisms. This causes the excretion of essential proteins and sugars from the body and further kidney damage. It takes a very long time before cadmium that has accumulated in kidneys is excreted from a human body's prolonged exposure.
Other health effects that can be caused by cadmium are:
- Diarrhoea, stomach pains and severe vomiting
- Bone fracture
- Reproductive failure and possibly even infertility
- Damage to the central nervous system
- Damage to the immune system
- Psychological disorders
- Possibly DNA damage or cancer development
Chemical properties of cadmium - Health effects of cadmium - Environmental effects of cadmium
http://www.lenntech.com/periodic/elements/cd.htm...
Chromium: Results = < 0.37
Chromium: pvl= 50 = results < 0.93
Chromium is an essential nutrient for humans and shortages may cause heart conditions, disruptions of metabolisms and diabetes, and exposure over a prolonged period of time. The uptake of too much chromium can cause health effects as well, for instance, skin rashes.
Chromium(VI) is a danger to human health, mainly to people who work in the steel and textile industry. People who smoke tobacco also have a higher chance of exposure to chromium.
Chromium(VI) is known to cause various health effects. When it is a compound in leather products, it can cause allergic reactions, such as skin rash. After breathing it in chromium(VI) can cause nose irritations and nosebleeds.
Other health problems that are caused by chromium(VI) are:
- Skin rashes
- Upset stomachs and ulcers
- Respiratory problems
- Weakened immune systems
- Kidney and liver damage
- Alteration of genetic material
- Lung cancer
- Death
Chemical properties of chromium - Health effects of chromium - Environmental effects of chromium
http://www.lenntech.com/periodic/elements/cr.htm...
Cobalt: Results < 0.2
Too high concentrations of cobalt may damage human health. When we breathe in too high concentrations of cobalt through air we experience lung effects, such as asthma and pneumonia. This mainly occurs with people who work with cobalt.
When plants grow on contaminated soils they will accumulate very small particles of cobalt, especially in the parts of the plant we eat, such as fruits and seeds. Soils near mining and melting facilities may contain very high amounts of cobalt so the uptake by humans through eating plants can cause health effects.
Health effects that are a result of the uptake of high concentrations of cobalt are:
- Vomiting and nausea
- Vision problems
- Heart problems
- Thyroid damage
Health effects may also be caused by the radiation of radioactive cobalt isotopes. This can cause sterility, hair loss, vomiting, bleeding, diarrhea, coma, and even death. This radiation is sometimes used with cancer patients to destroy tumors. These patients also suffer from hair loss, diarrhea, and vomiting.
Cobalt dust may cause an asthma-like disease with symptoms ranging from cough, shortness of breath, and dyspnea to decreased pulmonary function, nodular fibrosis, permanent disability, and death. Exposure to cobalt may cause weight loss, dermatitis, and respiratory hypersensitivity. LD 50 (oral, rat)- 6171 mg/kg. (LD50 = Lethal dose 50 = Single dose of a substance that causes the death of 50% of an animal population from exposure to the substance by any route other than inhalation. LD50 is usually expressed as milligrams or grams of material per kilogram of animal weight (mg/kg or g/kg).)
Carcinogenicity- The International Agency for Research on Cancer (IARC) has listed cobalt and cobalt compounds within group 2B (agents that are possibly carcinogenic to humans). ACGIH has placed cobalt and inorganic compounds in category A3 (Experimental animal carcinogen- the agent is carcinogenic in experimental animals at a relatively high dose, by route(s), histologic type(s), or by mechanism(s) that are not considered relevant to worker exposure.) Cobalt has been classified to be carcinogenic to experimental animals by the Federal Republic of Germany.
Chemical properties of cobalt - Health effects of cobalt - Environmental effects of cobalt
https://www.lenntech.com/periodic/elements/co.htm
Copper:
Copper can be found in many kinds of food, in drinking water, and in air. Because of that, we absorb eminent quantities of copper each day by eating, drinking, and breathing. The absorption of copper is necessary because copper is a trace element that is essential for human health. Although humans can handle proportionally large concentrations of copper, too much copper can still cause eminent health problems.
Copper concentrations in air are usually quite low so exposure to copper through breathing is negligible. But people that live near smelters that process copper ore into metal, do experience this kind of exposure.
People who live in houses that still have copper plumbing are exposed to higher levels of copper than most people because copper is released into their drinking water through the corrosion of pipes.
Occupational exposure to copper often occurs. In the working environment, copper contagion can lead to a flu-like condition known as metal fever. This condition will pass after two days and is caused by over-sensitivity.
Effects
Long-term exposure to copper can irritate the nose, mouth, and eyes and it causes headaches, stomachaches, dizziness, vomiting, and diarrhea. Intentionally high uptakes of copper may cause liver and kidney damage and even death. Whether copper is carcinogenic has not been determined yet.
Chemical properties of copper - Health effects of copper - Environmental effects of copper
https://www.lenntech.com/periodic/elements/cu.htm
Gallium:
Results = <0.32
Health effects of gallium
Gallium is an element found in the body, but it occurs in a very small amount. For example, in a person with a mass of seventy kilograms, there are 0.7 milligrams of gallium in the body. If this amount of gallium was condensed into a cube, the cube would only be 0.49 millimeters long on one side. It has no proven benefit towards the function of the body, and it most likely is only present due to small traces in the natural environment, in water, and in residue on vegetables and fruits. Several vitamins and commercially distributed waters have been known to contain trace amounts of gallium with less than one part per million. Pure gallium is not a harmful substance for humans to touch. It has been handled many times only for the simple pleasure of watching it melt by the heat emitted from a human hand. However, it is known to leave a stain on the hands. Even the gallium radioactive compound, gallium [67Ga] citrate, can be injected into the body and used for gallium scanning without harmful effects. Although it is not harmful in small amounts, gallium should not be purposefully consumed in large doses. Some gallium compounds can actually be very dangerous, however. For example, acute exposure to gallium(III) chloride can cause throat irritation, difficulty breathing, and chest pain, and its fumes can cause even very serious conditions such as pulmonary edema and partial paralysis.
Chemical properties of gallium - Health effects of gallium - Environmental effects of gallium
https://www.lenntech.com/periodic/elements/ga.htm
Iron: Pvl =200…Results 550.3
Iron in water is not a health hazard by itself but it may increase the hazard of pathogenic organisms since many of these organisms require iron to grow. 200 pvl Results 550.3 which is a staggering amount
Symptoms, signs, and diseases resulting from too much iron (iron overload):
§ chronic fatigue
§ joint pain
§ abdominal pain
§ liver disease (cirrhosis, liver cancer)
§ diabetes mellitus
§ Irregular heart rhythm
§ Heart attack or heart failure
§ Skin color changes (bronze, ashen-gray green)
§ Loss of period
§ loss of interest in sex
§ osteoarthritis
§ osteoporosis
§ hair loss
§ enlarged liver or spleen
§ impotence
§ infertility
§ hypogonadism
§ hypothyroidism
§ hypopituitarism
§ depression
§ Adrenal function problems
§ Early onset neurodegenerative disease
§ Elevated blood sugar
§ Elevated liver enzymes
§ Elevated iron (serum iron, serum ferritin)
Chemical properties of iron - Health effects of iron - Environmental effects of iron
https://www.lenntech.com/periodic/elements/fe.htm
Lead:
Signs of repeated lead exposure include:
• abdominal pain
• abdominal cramps
• aggressive behavior
• constipation
• sleep problems
• headaches
• irritability
• loss of developmental skills in children
• loss of appetite
• fatigue
• high blood pressure
• numbness or tingling in the extremities
• memory loss
• anemia
• kidney dysfunction
Since a child’s brain is still developing, lead can lead to intellectual disability. Symptoms may include:
• behavior problems
• low IQ
• poor grades at school
• problems with hearing
• short- and long-term learning difficulties
• growth delays
A high, toxic dose of lead poisoning may result in emergency symptoms. These include:
• severe abdominal pain and cramping
• vomiting
• muscle weakness
• stumbling when walking
• seizures
• coma
• encephalopathy, which manifests as confusion, coma, and seizures
Chemical properties of lead - Health effects of lead - Environmental effects of lead
https://www.lenntech.com/periodic/elements/pb.htm
Lithium;
Results = < 3.32 ug/L
For Bipolar Disorder to dumb you down
Lithium (Eskalith, Lithobid) is one of the most widely used and studied medications for treating bipolar disorder. Lithium helps reduce the severity and frequency of mania. It may also help relieve or prevent bipolar depression.
Studies show that lithium can significantly reduce suicide risk. Lithium also helps prevent future manic and depressive episodes. As a result, it may be prescribed for long periods (even between episodes) as maintenance therapy.
Lithium acts on a person's central nervous system (brain and spinal cord). Doctors don't know exactly how lithium works to stabilize a person's mood, but it is thought to help strengthen nerve cell connections in brain regions that are involved in regulating mood, thinking, and behavior.
Common side effects of lithium can include:
Hand tremors, Increased thirst, Increased urination, Diarrhea, Vomiting, Weight gain, Impaired memory, Poor concentration, Drowsiness, Muscle weakness, Hair loss, Acne, Decreased thyroid function, fever, unsteady walking, fainting, confusion, slurred speech, or rapid heart rate.
Chemical properties of lithium - Health effects of Lithium - Environmental effects of Lithium
https://www.lenntech.com/periodic/elements/li.htm
Manganese: Pvl=50… Results 14.78 ug/L
Health effects of manganese
Manganese is a very common compound that can be found everywhere on earth. Manganese is one out of three toxic essential trace elements, which means that it is not only necessary for humans to survive, but it is also toxic when too high concentrations are present in a human body. When people do not live up to the recommended daily allowances their health will decrease. But when the uptake is too high health problems will also occur.
The uptake of manganese by humans mainly takes place through food, such as spinach, tea, and herbs. The foodstuffs that contain the highest concentrations are grains and rice, soya beans, eggs, nuts, olive oil, green beans, and oysters. After absorption in the human body manganese will be transported through the blood to the liver, the kidneys, the pancreas, and the endocrine glands.
Manganese effects occur mainly in the respiratory tract and in the brain. Symptoms of manganese poisoning are hallucinations, forgetfulness, and nerve damage. Manganese can also cause Parkinson's, lung embolism, and bronchitis. When men are exposed to manganese for a longer period they may become impotent.
A syndrome that is caused by manganese has symptoms such as schizophrenia, dullness, weak muscles, headaches, and insomnia.
Because manganese is an essential element for human health shortages of manganese can also cause health effects. These are the following effects:
- Fatness
- Glucose intolerance
- Blood clotting
- Skin problems
- Lowered cholesterol levels
- Skeleton disorders
- Birth defects
- Changes of hair color
- Neurological symptoms
Chronic Manganese poisoning may result from prolonged inhalation of dust and fume. The central nervous system is the chief site of damage from the disease, which may result in permanent disability. Symptoms include languor, sleepiness, weakness, emotional disturbances, spastic gait, recurring leg cramps, and paralysis. A high incidence of pneumonia and other upper respiratory infections has been found in workers exposed to dust or fumes of Manganese compounds. Manganese compounds are experimental equivocal tumorigenic agents.
Chemical properties of manganese - Health effects of manganese - Environmental effects of manganese
http://www.lenntech.com/periodic/elements/mn.htm...
Nickel: Health effects of nickel
Nickel is a compound that occurs in the environment only at very low levels. Humans use nickel for many different applications. The most common application of nickel is its use as an ingredient in steel and other metal products. It can be found in common metal products such as jewelry.
Foodstuffs naturally contain small amounts of nickel. Chocolate and fats are known to contain severely high quantities. Nickel uptake will boost when people eat large quantities of vegetables from polluted soils. Plants are known to accumulate nickel and as a result, the nickel uptake from vegetables will be eminent. Smokers have a higher nickel uptake through their lungs. Finally, nickel can be found in detergents.
Humans may be exposed to nickel by breathing air, drinking water, eating food that it has fallen on or smoking cigarettes. Skin contact with nickel-contaminated soil or water may also result in nickel exposure. In small quantities nickel is essential, but when the uptake is too high it can be a danger to human health.
An uptake of too large quantities of nickel has the following consequences:
- Higher chances of development of lung cancer, nose cancer, larynx cancer and prostate cancer
- Sickness and dizziness after exposure to nickel gas
- Lung embolism
- Respiratory failure
- Birth defects
- Asthma and chronic bronchitis
- Allergic reactions such as skin rashes, mainly from jewelry
- Heart disorders
Nickel fumes are respiratory irritants and may cause pneumonitis. Exposure to nickel and its compounds may result in the development of a dermatitis known as “nickel itch” in sensitized individuals. The first symptom is usually itching, which occurs up to 7 days before skin eruption occurs. The primary skin eruption is erythematous, or follicular, which may be followed by skin ulceration. Nickel sensitivity, once acquired, appears to persist indefinitely.
Chemical properties of nickel - Health effects of nickel - Environmental effects of nickel
http://www.lenntech.com/periodic/elements/ni.htm...
Rubidium: Health effects of rubidium
Effects of exposure: water reactive. Moderately toxic by ingestion. If rubidium ignites, it will cause thermal burns. Rubidium readily reacts with skin moisture to form rubidium hydroxide, which causes chemical burns in the eyes and skin. Signs and symptoms of overexposure: skin and eye burns. Failure to gain weight, ataxia, hyper irritation, skin ulcers, and extreme nervousness. Medical conditions aggravated by exposure: heart patients, potassium imbalance.
First aid: Eye: immediately flush with running water for 15 minutes while holding eyelid. Obtain medical attention immediately. Skin: remove material and flush with soap and water. Remove contaminated clothing. Get medical attention promptly. Inhalation: move to fresh air immediately. If irritation persists, get medical attention. Ingestion: do not induce vomiting. Get medical attention immediately.
Chemical properties of rubidium - Health effects of rubidium - Environmental effects of rubidium
https://www.lenntech.com/periodic/elements/rb.htm
Selenium:
When selenium uptake is too high health effects will be likely to come about. The seriousness of these effects depends on the concentrations of selenium in the food and how often this food is eaten.
The health effects of various forms of selenium can vary from brittle hair and deformed nails to rashes, heat, swelling of the skin, and severe pains. When selenium ends up in the eyes people experience burning, irritation, and tearing.
Selenium poisoning may become so severe in some cases that it can even cause death.
Overexposure to selenium fumes may produce an accumulation of fluid in the lungs, garlic breath, bronchitis, pneumonitis, bronchial asthma, nausea, chills, fever, headache, sore throat, shortness of breath, conjunctivitis, vomiting, abdominal pain, diarrhea, and enlarged liver. Selenium is an eye and upper respiratory irritant and a sensitizer. Overexposure may result in red staining of the nails, teeth, and hair. Selenium dioxide reacts with moisture to form selenious acid, which is corrosive to the skin and eyes. Carcinogenicity- The International Agency for Research on Cancer (IARC) has listed selenium within Group 3 (The agent is not classifiable as to its carcinogenicity to humans.)
Chemical properties of selenium - Health effects of selenium - Environmental effects of selenium
https://www.lenntech.com/periodic/elements/se.htm
Silver: Results = <0.33 ug/L
No studies of cancer or birth defects in animals from eating, drinking, or breathing in silver compounds were found. Therefore, it is not known if these effects would occur in humans. One study of animals who drank silver compounds mixed with water for most of their lives found no effect on fertility. Another study found that reproductive tissues were damaged in animals after they received injections of silver nitrate. However, the tissue recovered even while the animals received more injections of silver nitrate. Tests in animals show that silver compounds are likely to be life-threatening for humans only when large amounts (that is, grams) are swallowed and that skin contact with silver compounds is very unlikely to be life-threatening.
Silver does have helpful uses. For example, silver nitrate was used for many years as drops in newborns' eyes to prevent blindness caused by gonorrhea, and it is also used in salves for burn victims. Some water treatment methods (including water filters) also use a form of silver to kill bacteria. Colloidal Silver is supposed to be very good for the human body.
Chemical properties of silver - Health effects of silver - Environmental effects of silver
https://www.lenntech.com/periodic/elements/ag.htm
Strontium 90: Side effects from in high doses that are in the Chem-trails
Strontium ranelate increases the risk of venous thromboembolism, pulmonary embolism serious cardiovascular disorders, and cancer including myocardial infarction. Its use is now restricted.[10] The most common side effects include nausea, diarrhea, headache, and eczema, but with only 2–4% increase compared with the placebo group. However, most of those side effects resolved within 3 months. Occasional severe allergic reactions have been reported including Drug Rash with Eosinophilia and Systemic Lupus Symptoms (DRESS syndrome
Thallium:
http://patient.info/doctor/thallium-poisoning
Epidemiology
Thallium poisoning is rare in Western societies yet we are being sprayed with it. It has occasionally been a tool for murder.
Thallium poisoning usually follows oral ingestion but it can be inhaled from contaminated dust from pyrite burners, cadmium manufacturing, lead and zinc smelting, and in contamination of heroin or cocaine. Its toxic effect is due to its ability to inhibit a number of intracellular potassium-mediated processes. It is used in the manufacture of electronic components, optical lenses, semiconductor materials, alloys, gamma radiation detection equipment, imitation jewelry, artists' paints, low-temperature thermometers, and green fireworks. In some parts of the world, it is still used for killing rodents and this may lead to inadvertent ingestion by humans.
The lethal dose is around 15-20 mg per kg body weight but serious toxicity and even death can occur with rather less. It is rapidly distributed throughout all tissues of the body. Most thallium is excreted by the fecal route but up to 35% may be excreted by the kidneys. Nevertheless, prolonged exposure may lead to build-up and chronic toxicity.
It was originally used for treating skin infections but had a low therapeutic index. Thallium isotopes are now used in some cardiac investigations but the dose is too low to be toxic.
Acute poisoning tends to produce gastrointestinal (GI) effects, whilst chronic poisoning tends to produce neurological manifestations. The classical triad of acute poisoning that should suggest the diagnosis of acute thallium toxicity is:
• Nausea and vomiting.
• Followed by painful peripheral neuropathy.
• Then alopecia.
Chemical properties of thallium - Health effects of thallium - Environmental effects of thallium
https://www.lenntech.com/periodic/elements/tl.htm
Tin:
Health effects of tin
Tin is mainly applied in various organic substances. Organic tin bonds are the most dangerous forms of tin for humans. Despite the dangers, they are applied in a great number of industries, such as the paint industry and the plastic industry, and in agriculture through pesticides. The number of applications of organic tin substances is still increasing, despite the fact that we know the consequences of tin poisoning.
The effects of organic tin substances can vary. They depend upon the kind of substance that is present and the organism that is exposed to it. Triethyltin is the most dangerous organic tin substance for humans. It has relatively short hydrogen bonds. When hydrogen bonds grow longer a tin substance will be less dangerous to human health. Humans can absorb tin bonds through food and breathing and through the skin.
The uptake of tin bonds can cause acute effects as well as long-term effects.
Acute effects are:
- Eye and skin irritations
- Headaches
- Stomachaches
- Sickness and dizziness
- Severe sweating
- Breathlessness
- Urination problems
Long-term effects are:
- Depressions
- Liver damage
- Malfunctioning of immune systems
- Chromosomal damage
- Shortage of red blood cells
- Brain damage (causing anger, sleeping disorders, forgetfulness and headaches)
Chemical properties of tin - Health effects of tin - Environmental effects of tin
http://www.lenntech.com/periodic/elements/sn.htm...
Uranium: KILLS YOU ..
Uranium Health Effects
A discussion of chemical and radiological health effects associated with exposure to uranium and its compounds.
Chemical Toxicity
Exposure to uranium can result in both chemical and radiological toxicity. The main chemical effect associated with exposure to uranium and its compounds is kidney toxicity. This toxicity can be caused by breathing air containing uranium dusts or by eating substances containing uranium, which then enters the bloodstream. Once in the bloodstream, the uranium compounds are filtered by the kidneys, where they can cause damage to the kidney cells. Very high uranium intakes (ranging from about 50 to 150 mg depending on the individual) can cause acute kidney failure and death. At lower intake levels (around 25 to 40 mg), damage can be detected by the presence of protein and dead cells in the urine, but there are no other symptoms. Also, at lower intake levels, the kidney repairs itself over several weeks after the uranium exposure has stopped.
Chemical properties of uranium - Health effects of uranium - Environmental effects of uranium
https://www.lenntech.com/periodic/elements/u.htm
Vanadium:
Health effects of vanadium
Vanadium compounds are not regarded as serious hazards, however, workers exposed to vanadium peroxide dust were found to suffer severe eye, nose, and throat irritation.
The uptake of vanadium by humans mainly takes place through foodstuffs, such as buckwheat, soya beans, olive oil, sunflower oil, apples, and eggs.
Vanadium can have a number of effects on human health when the uptake is too high. When vanadium uptake takes place through air it can cause bronchitis and pneumonia.
The acute effects of vanadium are irritation of the lungs, throat, eyes, and nasal cavities.
Other health effects of vanadium uptake are:
- Cardiac and vascular disease
- Inflammation of the stomach and intestines
- Damage to the nervous system
- Bleeding of livers and kidneys
- Skin rashes
- Severe trembling and paralysis
- Nose bleeds and throat pains
- Weakening
- Sickness and headaches
- Dizziness
- Behavioral changes
The health hazards associated with exposure to vanadium are dependent on its oxidation state. This product contains elemental vanadium. Elemental vanadium could be oxidized to vanadium pentoxide during welding. The pentoxide form is more toxic than the elemental form. Chronic exposure to vanadium pentoxide dust and fumes may cause severe irritation of the eyes, skin, and upper respiratory tract, persistent inflammations of the trachea and bronchi, pulmonary edema, and systemic poisoning. Signs and symptoms of overexposure include; conjunctivitis, nasopharyngitis, cough, labored breathing, rapid heartbeat, lung changes, chronic bronchitis, skin pallor, greenish-black tongue, and an allergic skin rash.
Chemical properties of vanadium - Health effects of vanadium - Environmental effects of vanadium
https://www.lenntech.com/periodic/elements/v.htm?#ixzz4CRxedIhf
At present, due to “location” restrictions, I am not capable of monetizing my substack.
So! for anyone who would like to help support my ongoing efforts, please make a deposit here -
paypal.me/AJHewett –
Thank You!
Much Needed, Much Appreciated
https://linktr.ee/hewettinsite